电化学原位红外测试
1. 几种原位电化学红外构造简介
利用分子振动的特征吸收,红外光谱可以用于原位检测固体电极上的吸附态物种,从而:(1) 关键中间产物的识别和构型鉴定(2)确定电极表面成键状态(3)优先的反应路径和选择性确认(4)探究反应环境的影响,如电解液阴离子,阳离子,pH, 添加剂等(5)电极电解液界面双电层的探测。适用于水系或非水系电催化反应,如CO2RR, HER, OER, ORR, MOR, EOR, NRR,硝酸根还原,有机电合成等。
1.1 IRAS
IRAS或IRRAS,也称为外反射模式,或透反射模式,光滑电极(玻碳电极,光滑金属电极或FTO等基底)或将电催化剂滴涂或电沉积到光滑电极上,压到红外窗口上形成一层电解液薄层(1-10微米),红外光束穿过光学窗口经过电解液薄层,然后在电极表面反射红外光,最后到达红外检测器。 外反射模一般选用CaF2窗片,窗片的形状一般为厚度2mm左右的圆片,梯形、半球形或半圆柱型的晶体也经常被使用。
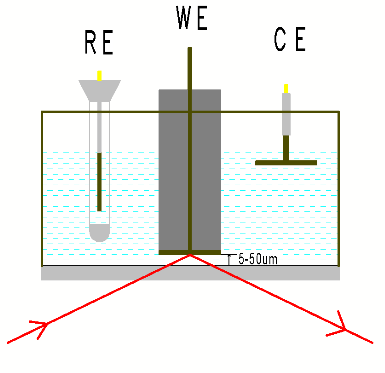
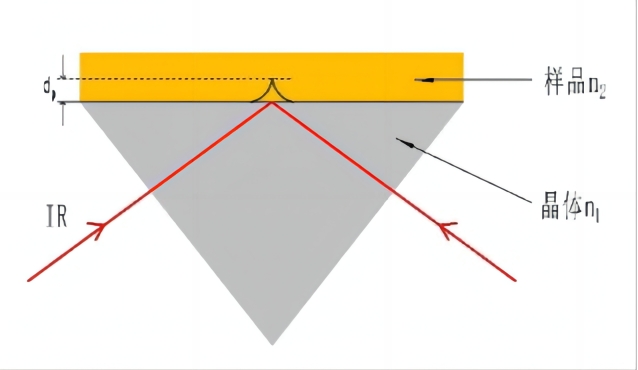
图1 外反射示意图 图2 ATR原理示意图
1.2 衰减全反射原理的理解
衰减全反射基本原理:红外光经过折射率大的晶体再入射到折射率小的试样表面上,当入射角大于临界角时,入射光线就会产生全反射。 事实上红外光并不是全部被反射回来,而是穿透到试样表面内一定深度后再返回表面在该过程中,试样在入射光频率区域内有选择吸收,反射光强度发生减弱,产生与透射吸收相类似图,从而获得样品表层化学成份的结构信息。 见图2 ATR原理示意图。 红外光穿透晶体界面的深度与晶体折射率和入射光角度有关,常见晶体的折射率见表2,折射率越大,穿透深度越小。
1.3 内反射ATR-SEIRAS和内反射Otto薄层模式
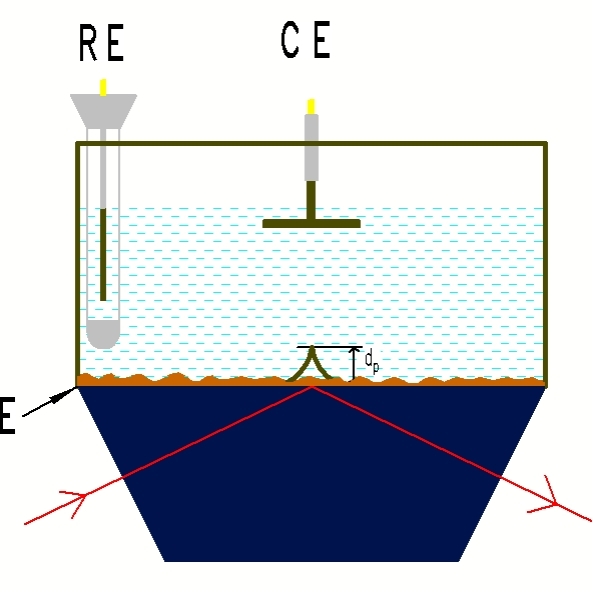
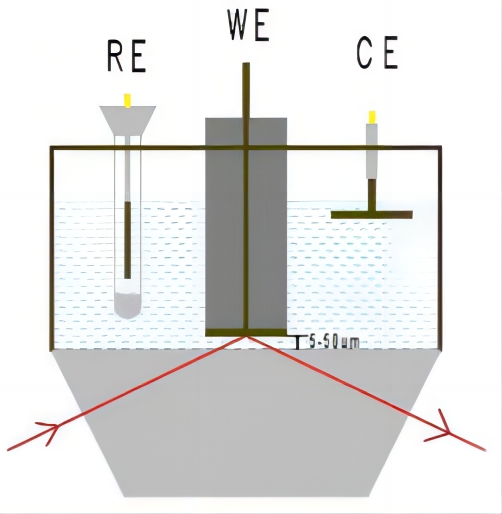
图3 内反射ATR-SEIRAS 图4 内反射Otto薄层模式
内反射ATR-SEIRAS模式,也称为Kretschmann构造,如上图所示,ATR晶体(ZnSe, Si, Ge)平面上化学镀或真空镀一层岛状金属膜,在金属膜上底涂或电沉积催化剂,金属膜作为导电基底,同时作为表面增强剂,使催化剂表面吸附分子的红外信号会比没有金属膜时的信号增强10-1000倍。 这称为表面增强红外效应,即SEIRAS。 不同晶体耐受不同范围pH,光谱范围也不同,详见表2
内反射Otto薄层模式,与外反射构造类似,区别是内反射Otto薄层模式下一般选用ZnSe,Ge,Si,金刚石等衰减全反射晶体,是基于ATR的反射原理,见图4。
2. 实验注意事项
2.1 内外反射的选择依据
电化学池
| 可选晶体 | 传质情况 | 电场线分布情况 | 电极材料要求 | 可检测物种 |
ATR内反射Kretschmann(ATR-SEIRAS) | Diamond,Si,Ge, ZnSe | 非薄层构造,传质阻力小 | 均匀 | 金属膜+滴涂或电沉积催化剂 | 吸附态 |
ATR内反射Otto薄层模 式 | Diamond,Si,Ge, ZnSe | 薄层构造,传质阻力大 | 不均匀 | 镜面光滑或粗糙表面催化剂(如碳纸或泡沫镍负载型) | 吸附态+溶液相 |
外反射薄层模式(IRRAS) | CaF2 | 薄层构造,传质阻力大 | 不均匀 | 玻碳电极等镜面光滑基底电极+滴涂或电沉积催化剂 | 吸附态+溶液相 |
表1 电化学原位红外光谱电化学池构型对比
2.2 晶体选择
晶体种类 | 光谱范围(cm-1) | pH 范围 | 折射率(折射率越大,穿透深度越小) |
ATR模式晶体 | |||
Diamond | 525-4000 | 1-14 | 2.42 |
ZnSe | 520-4000 | 5-9 | 2.40 |
Si | 1200-4000 | 1-12 | 3.40 |
Ge | 575-4000 | 1-14 | 4.00 |
外反射模式晶体 | |||
CaF2 | 1100-4000 | 5-8 | 1.43 |
表2 不同晶体物理化学特性
2.3 入射角度
在ATR和外反射测量中,入射角的角度和是否选用偏振光作为光源决定了得到的光谱强度; 理论上,大的入射角度(70-80°)会更好一些。 然而,由于光束在界面上沿着入射角度并且一般电极尺寸在1-2cm直径,当角度在60-80°范围内改变时,没有发现明显的信号增强。 使用60或65°的光路,相同的光路附件可以用于外反射和内反射ATR-SEIRAS。
2.4 正峰和倒峰
红外光谱谱图的展示有吸光度和透过率两种方式,互为向上和向下的关系,便于讨论,假定以吸光度纵坐标,对于某个电位下的参考谱图(通常为一条直线),随电位变化,如果产生正峰(即向上),认为是某种物种生成,若为倒峰(即向下),表示某种物种消耗了。如果针对特定的反应,当某种物种消耗的倒峰解释不通,或者某个范围内的峰一上一下,谱峰出现异常的时候,可能与其他两个因素有关,(1)入射角度(2)金属颗粒(SEIRAS基底或纳米催化剂颗粒)的体积填充因子(volume fraction of the composite occupied by the metal particle),注意:不是颗粒的尺寸。
2.5 光路的选择
目前常用的光路有两种,一种是入射角度(一般为60-70度)固定的光路附件,另一种是入射角度可调节(30-80度)的光路附件。可调角度的光路附件,由于采用了平面镜和非平面的聚焦镜组合,增加了镜片的数量从而增大了光能量的损失,采用一次反射的附件的光路系统,由于没有多次反射减少了能量的损失,得益于高灵敏度的MCT检测器,两种光路均可得到高质量的光谱谱图。
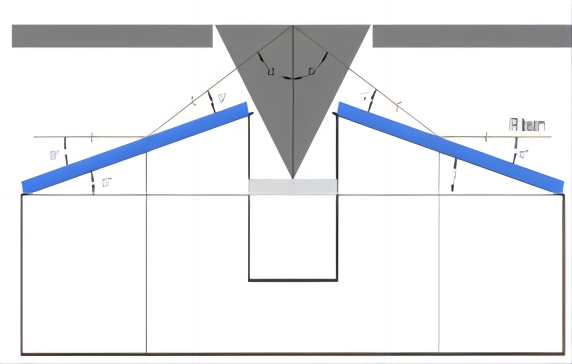
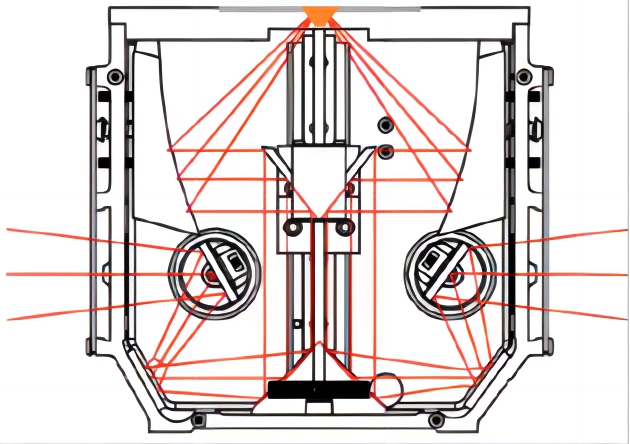
图5 60°入射角光路示意图 图6 VeeMaxIII光路示意图
2.6 电化学池的选择(单腔室和双腔室,流动和静止)
电化学池的种类非常多,有单腔室的(即三电极处在同一腔室内)和双腔室的。Dunwell等人[4]系统研究了电解液纯度和对电极的种类,研究结果推荐:(1)对于CO2电还原,推荐采用双腔室,质子交换膜将对电极和工作电极隔开,以避免对电极产物扩散到工作电极干扰工作电极的反应(2)采用高纯度试剂配置电解液,有时候需要纯化电解液(3)对电极采用碳棒,不采用Pt丝。因为Pt会溶解,可能扩散到阴极,Pt是良好的析氢催化剂。Malkani等人详细对比了流动和静止CO电还原效果,研究表明搅拌的情况下传质更好。但是需要注意的是,实际操作中有些催化剂在强烈对流的状态下会从晶体表面脱落,导致实验无法进展。
ATR-SEIRAS模式,或Kretschmann模式,仅适用于粉末催化剂或在金属膜上电沉积其他催化剂,对于大块电极或碳纸等多孔基底的催化剂无法表征,因此外反射或Otto模式的也偶尔有使用到。
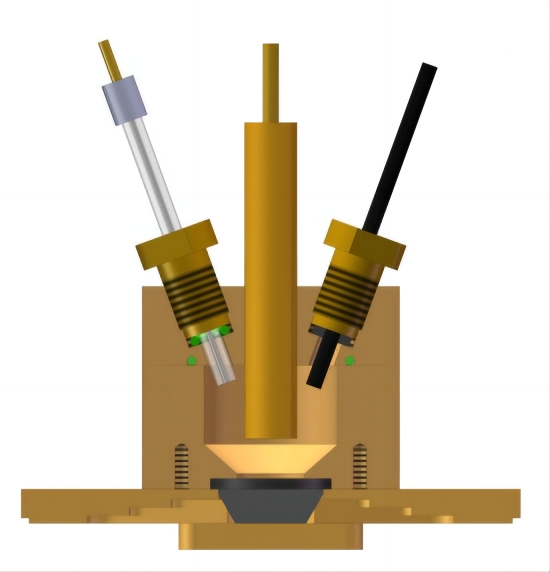
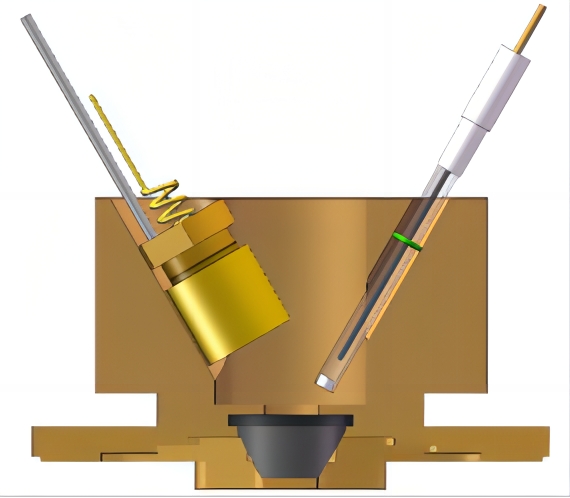
图7 外反射电化学池 图8 小体积内反射双腔室电化学池
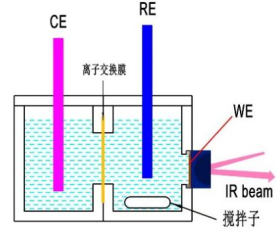
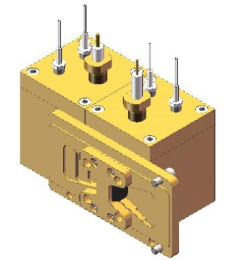
图9 双腔室可搅拌电化学池示意图 图10 双腔室可搅拌电化学池
2.7 表面增强红外注意事项
(1)光谱增强的强度依赖于金属的表面形貌,真空蒸镀和电化学沉积所制备的金属岛状膜都能得到很好的增强效果;
(2)物理吸附和化学吸附的分子都能得到增强,一般情况下化学吸附的分子的增强强度要比物理吸附的分子强;
(3)与金属表面直接相连的第一层分子的信号最强,增强效应随着电极表面距离的增加而衰减,即表面增强是一种短程效应。
SEIRAS基底的制备
许多材料可以用来作为金属沉积的基底,如Ge,Si,ZnSe,CaF2等。增强因子一般和基底材料的折射率以及基底材料本身的化学性质有关,基底本身的化学特性是影响增强效果的很重要因素,因为它决定沉积膜的表面形貌。在每次沉积前对基底的打磨和清洗是保证实验重复性的管件因素,有时候基底的表面修饰也影响增强效果。
(1)干法制备纳米薄膜
主要有真空蒸镀和溅射方式。金属膜的增强效果受形貌,颗粒大小,岛状颗粒密度等因素影响。对于蒸镀,比较低的沉积速率(0.1-0.5nm/min)有利于较好的增强效果。
(2)湿法制备纳米薄膜
化学镀制备得到的金属膜比真空镀的膜岛状纳米离子的粒径要大,化学沉积制备的Au膜与硅基底之间的粘附力要比真空蒸镀的大得多,而且SEIRAS增强因子更大,这可能是因为基底和金膜之间不存在氧化物,即化学镀之前硅表面先用NH4F去除了表面氧化物。另外,增强的电场会在氧化层内发生衰减,因此制备好的金属膜要尽快使用,或者使用前进行电化学清洗。
2.8 电化学测试方法的选择
原位电化学红外光谱检测中常用的电化学方法有LSV/CV,计时电流法,单步电位阶跃法,多步骤电位阶跃法,方波伏安法等。根据所选择的电化学方法相应的光谱数据采集有手动采集和自动采集两种。
3. 部分应用案例
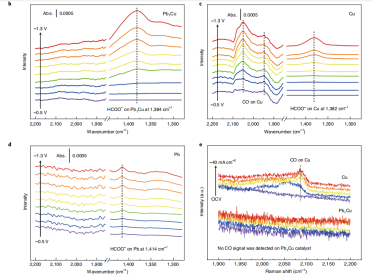
CO2电还原Nature Nanotechnology 2021,16, 1386–1393
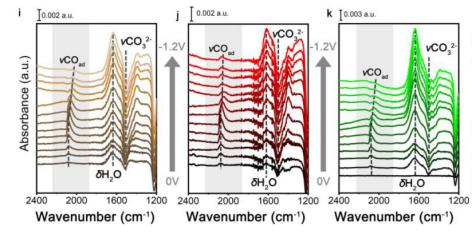
CO2电还原 J. Am. Chem. Soc.2022, 144, 259−269
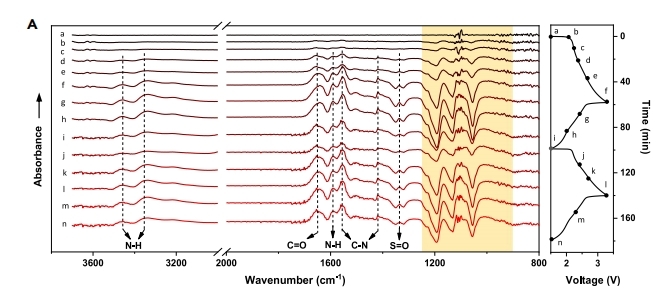
锌离子电池 Joule 2022, 6, 399–417
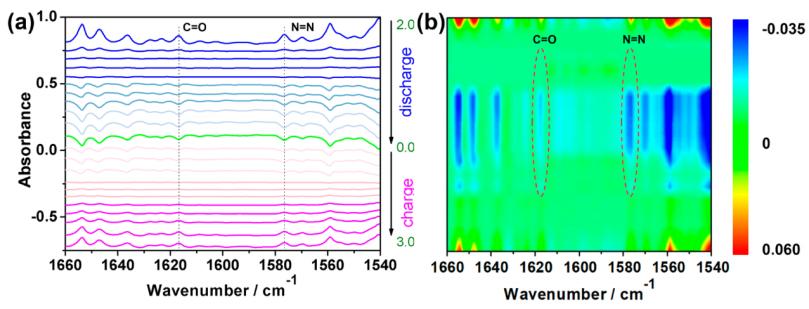
锂离子电池ACS Energy Lett. 2020, 5, 1022−1031
4. 部分客户论文发表清单:
Jianping Xiao*, Bin Zhang*, et al. Unveiling hydrocerussite as an electrochemically stable active phase for efficient carbon dioxide electroreduction to formate. Nat. Commun. 2020, 11, 3415
Lei Yan, Yonggang Wang*, et al. Chemically Self-Charging Aqueous Zinc-Organic Battery. J. Am. Chem. Soc. 2021, 143, 15369-15377
Nan Wang, Yonggang Wang*, et al. Zinc-organic Battery with a Wide Operation-temperature Window from -70 to 150 oC Angew. C.hem. Int. Ed. 2020,59,14577-14583
Bingliang Wang, Yongyao Xia*, et al. In situ structural evolution of the multi-site alloy electrocatalyst to manipulate the intermediate for enhanced water oxidation reaction. Energy Environ. Sci. 2020, 13, 2200-2208
Yang Peng*, et al. Breaking Linear Scaling Relationship by Compositional and Structural Crafting of Ternary Cu-Au/Ag Nanoframes for Electrocatalytic Ethylene Production. Angew. Chem. Int. Ed. 2021, 60, 2508-2518
Zhuo Yu, Yonggang Wang*, et al. Boosting Polysulfide Redox Kinetics by Graphene-Supported Ni Nanoparticles with Carbon Coating. Adv. Energy Mater. 2020, 10, 2000907
Xinwei Ding, Zhi Yang*, et al. Biomimetic Molecule Catalysts to Promote the Conversion of Polysulfides for Advanced Lithium–Sulfur Batteries Adv. Funct. Mater. 2020, 30, 2003354
Hong Guo*, Xueliang Sun*, et al. Dual Active Site of the Azo and Carbonyl-Modified Covalent Organic Framework for High-Performance Li Storage. ACS Energy Lett. 2020, 5, 1022-1031
Suya Zhou, Zhi Yang*, et al. Dual-Regulation Strategy to Improve Anchoring and Conversion of Polysulfides in Lithium–Sulfur Batteries ACS Nano 2020, 14, 7538–7551
Yongyao Xia*, et al. Low-Temperature Charge/Discharge of Rechargeable Battery Realized by Intercalation Pseudocapacitive Behavior. Adv. Sci. 2020, 7, 2000196
Lei Wang*, Yonggang Wang, et al. Pencil-drawing on nitrogen and sulfur co-doped carbon paper: An effective and stable host to pre-store Li for high-performance lithium–air batteries. Energy Storage Materials 2020, 26, 593-603
Guanglei Cui*, Liquan Chen, et al. Non-flammable nitrile deep eutectic electrolyte enables high voltage lithium metal batteries. Chem. Mater. 2020, 32, 3405-3413
Guanglei Cui*, et al. Investigation on the Cathodic Interfacial Stability of Nitrile Electrolyte and its performance with High Voltage LiCoO2 Chem. Commun. 2020, 56, 4998-5001
Zhongbin Zhuang*, et al. A highly-active, stable and low-cost platinum-free anode catalyst based on RuNi for hydroxide exchange membrane fuel cells. Nat. Commun. 2020, 11, 5651
Tiancun Liu, Yong Wang*, et al. Organic supramolecular protective layer with rearranged and defensive Li deposition for stable and dendrite-free lithium metal anode. Energy Storage Materials 2020, 32, 261–271
X. Yin, Y. Wang*, et al. Designing cobalt-based coordination polymers for high-performance sodium and lithium storage: from controllable synthesis to mechanism detection. Materials Today Energy 2020, 17, 100478
Song Chen, Jintao Zhang*, et al. Regulation of Lamellar Structure of Vanadium Oxide via Polyaniline Intercalation for High-Performance Aqueous Zinc-Ion Battery. Adv. Funct. Mater. 2020, 30, 2003890
Nannan Meng, Yifu Yu, Bin Zhang*, et al. Efficient Electrosynthesis of Syngas with Tunable CO/H2 Ratios over ZnxCd1-xS-Amine Inorganic-Organic Hybrids. Angew. Chem. Int. Ed. 2019, 58, 18908–18912
Yanrong Xue, Zhongbin Zhuang*, et al. Sulfate-Functionalized RuFeOx as Highly Efficient Oxygen Evolution Reaction Electrocatalyst in Acid. Adv. Funct. Mater. 2021, 31, 2101405
Hong Guo*, et al. Cooperative catalytic interface accelerates redox kinetics of sulfur species for high-performance Li-S batteries. Energy Storage Materials 2021, 40, 139-149
Yang Peng*, et al. Geometric Modulation of Local CO Flux in Ag@Cu2O Nanoreactors for Steering the CO2RR pathway toward High-Efficacy Methane Production. Adv. Mater. 2021, 33, 2101741
Yonggang Wang*, et al. Molecular Tailoring of n/p-type Phenothiazine Organic Scaffold for Zinc Batteries. Angew. Chem. Int. Ed. 2021, 60, 20826-20832
Hongliang Jiang*, Chunzhong Li*, et al. Dynamically Formed Surfactant Assembly at the Electrified Electrode–Electrolyte Interface Boosting CO2 Electroreduction. J. Am. Chem. Soc. 2022, 144, 6613–6622
Yang Peng*, et al. Au-activated N motifs in non-coherent cupric porphyrin metal organic frameworks for promoting and stabilizing ethylene production. Nat. Commun. 2022, 13, 63
Jie Zeng*, et al. Copper-catalysed exclusive CO2 to pure formic acid conversion via single-atom alloying. Nature Nanotechnology 2021, 16, 1386-1393
Min-Rui Gao*, et al. Identification of Cu(100)/Cu(111) Interfaces as Superior Active Sites for CO Dimerization During CO2 Electroreduction. J. Am. Chem. Soc. 2022, 144, 1, 259-269
Chen Feng, Shiming Zhou*, Jie Zeng*, et al. Tuning the Electronic and Steric Interaction at the Atomic Interface for Enhanced Oxygen Evolution. J. Am. Chem. Soc. 2022, 144,21,9271-9279
Rui Lin, Jianhui Wang, et al. Asymmetric donor-acceptor moleculeregulated core-shell-solvation electrolyte for high-voltage aqueous batteries. Joule 2022, 6, 399–417
Zhongju Wang, Yongzhu Fu*, et al. Biredox‐Ionic Anthraquinone‐Coupled Ethylviologen Composite Enables Reversible Multielectron Redox Chemistry for Li‐Organic Batteries. Adv. Sci. 2022, 9, 2103632
Jintao Zhang*, et al. Defect evolution of hierarchical SnO2 aggregatesfor boosting CO2 electrocatalytic reduction. J. Mater. Chem. A 2021, 9, 14741-14751
Fei Ai, Yijun Lu*, et al. Heteropoly acid negolytes for high-power-density aqueous redox flow batteries at low temperatures. Nature Energy. 2022, 7, 417–426
Zhejun Li, Yijun Lu*. Polysulfide-based redox flow batteries with long life and low levelized cost enabled by charge-reinforced ion-selective membranes. Nature Enery. 2021, 6, 517–528
Tieliang Li, Yifu Yu, Bin Zhang*, et al. Sulfate-Enabled Nitrate Synthesis from Nitrogen Electrooxidation on Rhodium Electrocatalyst. Angew. Chem. Int. Ed. 2022, e202204541
Yanbo Li, Bin Zhang, Yifu Yu*, et al. Electrocatalytic Reduction of Low-Concentration Nitric Oxide into Ammonia over Ru Nanosheets. ACS Energy Letters. 2022, 7, 1187-1194
Yanmei Huang, Yifu Yu, Bin Zhang*, et al. Direct Electrosynthesis of Urea from Carbon Dioxide and Nitric Oxide. ACS Energy Letters. 2022, 7, 284-291
Wenfu Xie, Hao Li, Min Wei*, et al. NiSn Atomic Pair on Integrated Electrode for Synergistic Electrocatalytic CO2 Reduction. Angew. Chem. Int. Ed. 2021, 60, 7382–7388
Rui Sui, Jiajing Pei, Zhongbin Zhuang*, et al. Engineering Ag−Nx Single-Atom Sites on Porous Concave N Doped Carbon for Boosting CO2 Electroreduction. ACS Appl. Mater. Interfaces. 2021, 13, 17736-17744
Tiliang Li, Yuting Wang, Yifu Yu*, Bin Zhang*, et al. Ru-Doped Pd Nanoparticles for Nitrogen Electrooxidation to Nitrate. ACS Catal. 2021, 11, 14032-14037
Jintao Zhang* et al. Atomic Bridging Structure of Nickel-Nitrogen-Carbon for Highly Efficient Electrocatalytic Reduction of CO2. Angew. Chem.Int. Ed. 2022, 61, e202113918
Lang Xu* et al. Gadolinium Changes the Local Electron Densities of Nickel 3d Orbitals for Efficient Electrocatalytic CO2 Reduction. Angew. Chem.Int. Ed. 2022, 61, e202201166
Bin Zhang* et al. Phenanthrenequinone-like moiety functionalized carbon for electrocatalytic acidic oxygen evolution. Chem. 2022, 8, 1415-1426
Sheng Dai*, Minghui Zhua*, Yifan Han* et al. Probing the role of surface hydroxyls for Bi, Sn and In catalysts during CO2 Reduction. Applied Catalysis B: Environmental. 2021, 298,

